 |
How to Balance Aluminum + Nitric acid → Aluminum nitrate + Hydrogen gas |
How to Balance: Al + HNO3 → Al(NO3)3 + H2
Type of Chemical Reaction: This is a Sin reaction.
Word Equation: Aluminum + Nitric acid → Aluminum nitrate + Hydrogen gas
| Balancing Strategies: In this single displacement reaction the Al and H are switching places. To balance the equation, consider the nitrate ion (NO3-) to be one item. That will facilitate balancing.
This is a bit of a challenging equation to balance! For a complete explanation, watch:
About Balancing Chemical EquationsWhen balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.Only change the coefficients (these are the numbers in front substances).
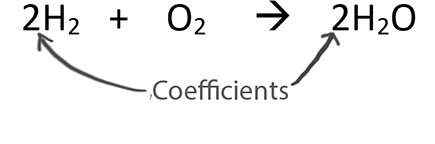 Never change the subscripts (the small numbers after elements).
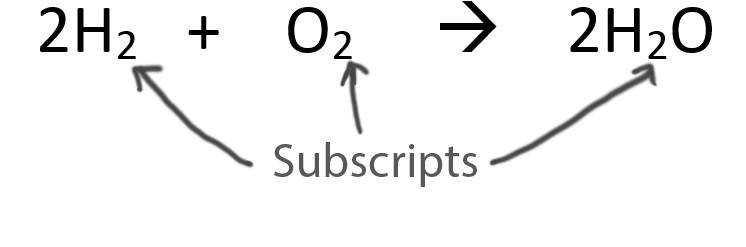 Video:Subscribe Back to the List of Equations |