 |
How to Balance Potassium hydroxide + Carbon dioxide → Potassium bicarbonate |
How to Balance: KOH + CO2 → KHCO3
Type of Chemical Reaction: This is a combination reaction.
Word Equation: Potassium hydroxide + Carbon dioxide → Potassium bicarbonate
| Balancing Strategies: In this combination (also called synthesis reaction) we have KOH and CO2 combining to form KHCO3.
Be sure to count all of the oxygen atoms on the reactants side of the equation. For a complete explanation, watch:
About Balancing Chemical EquationsWhen balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.Only change the coefficients (these are the numbers in front substances).
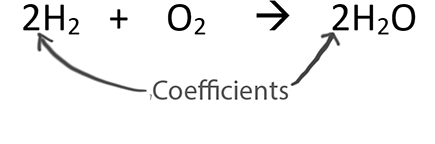 Never change the subscripts (the small numbers after elements).
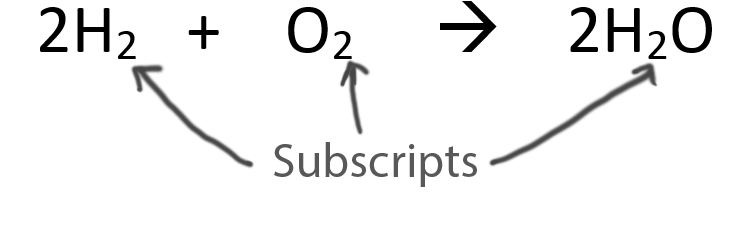 Video:Subscribe Back to the List of Equations |