 |
How to Balance Sodium hydrogen carbonate + Acetic acid → Sodium acetate + Carbon dioxide + Water |
How to Balance: NaHCO3 + HC2H3O2 → NaC2H3O2 + CO2 + H2O
Type of Chemical Reaction: This is a chemical reaction.
Word Equation: Sodium hydrogen carbonate + Acetic acid → Sodium acetate + Carbon dioxide + Water
| Balancing Strategies: In this reaction we have NaHCO3 (baking soda) reacting with an aqueous solution of HC2H3O2 (vinegar) to form NaC2H3O2 + CO2 + H2O.
The bubbles you see when you mix backing soda with vinegar are the CO2 found on the product side of the equation. For a complete explanation, watch:
About Balancing Chemical EquationsWhen balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.Only change the coefficients (these are the numbers in front substances).
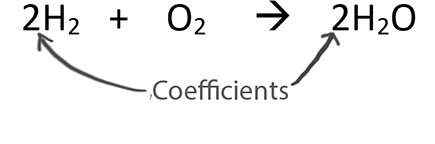 Never change the subscripts (the small numbers after elements).
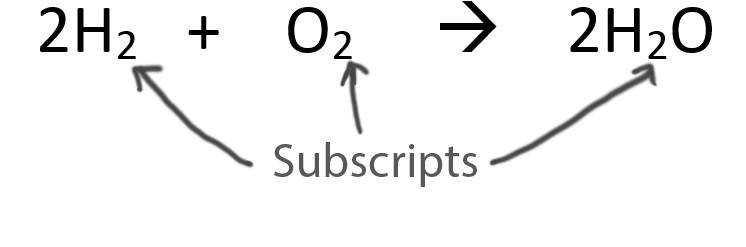 Video:Subscribe Back to the List of Equations |